Memorandum D19-9-4: Importation and Exportation of Pathogens and Toxins
ISSN 2369-2391
Ottawa, October 26 2023
This document is also available in PDF (788 KB) [help with PDF files]
The purpose of this D-memorandum is to explain the requirements for importers and exporters that wish to import and export pathogens and toxins.
On this page
- Definitions and acronyms
- Guidelines
- Importing pathogens or toxins
- Exporting pathogens or toxins
- Exemptions
- Exclusions
- Role of the CBSA
- Penalties
- Appendix A: Samples of permits and licences
- References
- Contact us
- Related links
Definitions and acronyms
The following acronyms and definitions apply to this memorandum:
Acronyms
- AG
- means Australia Group
Note: The Australia Group (AG) was established in an effort to prevent the proliferation of chemical and biological weapons. State participants of the AG have developed common export controls on chemical substances and biological agents and related items that could be used in the production of chemical and biological weapons. These export controls have been implemented in Canada on the Export Control List as Group 7.
- BSO
- means Border Services Officer
- CBSA
- means Canada Border Services Agency
- CFIA
- means Canadian Food Inspection Agency
- ECL
- means Export Control List: the ECL was implemented to fulfill Canada's international commitments under the Convention on the Prohibition of the Development, Production and Stockpiling of Bacteriological (Biological) and Toxin Weapons and on their Destruction, commonly known as the Biological and Toxin Weapons Convention (BTWC), among other commitments.
- GAC
- means Global Affairs Canada
- HAA
- means Health of Animals Act
- HAR
- means Health of Animals Regulations
- HPTA
- means Human Pathogens and Toxins Act
- HPTR
- means Human Pathogens and Toxins Regulations
- PHAC
- means Public Health Agency of Canada
- SSBA
- means security sensitive biological agents; SSBAs, are a subset of Risk Group 3 and 4 human pathogens and prescribed toxins that are included in Schedule 1 of the Human Pathogens and Toxins Act
Definitions
- Controlled activity
- means an activity referred to in subsection 7(1) of the HPTA except when already subject to the Export and Import Permits Act or the Transportation of Dangerous Goods Act, 1992. These include the following: possessing, handling or using a human pathogen or toxin; producing a human pathogen or toxin; storing a human pathogen or toxin; permitting any person access to a human pathogen or toxin; transferring a human pathogen or toxin; importing or exporting a human pathogen or toxin; releasing or otherwise abandoning a human pathogen or toxin; or disposing of a human pathogen or toxin.
- Import permit
- A permit issued by the Canadian Food Inspection Agency (CFIA) under the HAA and HAR for the importation of animal pathogens including animal pathogens causing an emerging or foreign animal disease, bee and aquatic animal pathogens, animal pathogens contained in animals, animal products, animal by-products or other organisms carrying an animal pathogen or part of one.
- Licence
A document consisting of a Pathogen and Toxin Licence:
- a) issued by PHAC under section 18 of the HPTA authorizing the conduct of one or more controlled activities with human pathogens or toxins.
and/or
- b) issued by PHAC under section 160 of the HAR authorizing the importation of terrestrial animal pathogens or toxins as defined under section 51 (a) of the HAR other than animal pathogens that cause foreign animal diseases or emerging animal diseases, bee and aquatic animal pathogens, and animal pathogens in animal products, and authorizing the domestic transfer of the imported material as defined under section 51.1 (a)(b) of the HAR.
- a) issued by PHAC under section 18 of the HPTA authorizing the conduct of one or more controlled activities with human pathogens or toxins.
- Pathogen
- means a microorganism, nucleic acid, or protein capable of causing disease or infection in humans or animals. Examples of human pathogens are listed in Schedules 2 to 4 and in Part 2 of Schedule 5 of the Human Pathogens and Toxins Act, but these are not exhaustive lists.
- Animal pathogen
- means any pathogen that causes disease in animals; including those derived from biotechnology.
- Aquatic animal pathogen
- means a pathogen or part of one that causes diseases in aquatic animals. In the context of animal pathogens, this includes aquatic animals (for example, finfish, mollusc, crustacean), including mammals (for example, seals, dolphins) which spend their lives in water.
- Human pathogen
means a micro-organism, nucleic acid or protein that
- (a) is listed in any of Schedules 2 to 4 or in Part 2 of Schedule 5 of HPTA; or
- (b) is not listed in any of the Schedules but falls into Risk Group 2 (RG2), Risk Group 3 (RG3) or Risk Group 4 (RG4).
- Plant pathogen
- means a pathogen that is injurious to plants.
- Terrestrial animal pathogen
- means a pathogen or part of one that causes diseases in terrestrial animals, including avian and amphibian animals, but excluding aquatic animals and invertebrates.
- Zoonotic pathogen
- means pathogen that causes disease in humans and animals, and that can be transmitted from animals to humans and vice versa (i.e., zoonoses). They are considered both human and animal pathogens.
- Person
- means an individual or an organization as defined in section 2 of the Criminal Code.
- Produce
in respect of a human pathogen or toxin, means to create it by any method or process, including
- (a) by manufacturing, cultivating, developing, reproducing or synthesizing it; or
- (b) by converting or refining a substance, micro-organism, nucleic acid or protein, or by using any other means of altering its physical or chemical properties.
- Release
- means any discharge, anywhere, and includes leaking, spraying, depositing, dumping or vaporizing.
- Risk Group 2
- means a category of pathogens that pose a moderate risk to the health of individuals or animals and a low risk to public health. They are able to cause serious disease in a human but are unlikely to do so. Effective treatment and preventive measures are available and the risk of spread of disease caused by those pathogens is low. Examples of RG2 human pathogens are listed in Schedule 2 in the HPTA.
- Risk Group 3
- means a category of pathogens that pose a high risk to the health of individuals or animals and a low risk to public health. They are likely to cause serious disease in a human. Effective treatment and preventive measures are usually available and the risk of spread of disease caused by those pathogens is low. Examples of RG3 human pathogens are listed in Schedule 3 of HPTA
- Risk Group 4
- means a category of pathogens that pose a high risk to the health of individuals or animals and a high risk to public health. They are likely to cause serious disease in a human. Effective treatment and preventive measures are not usually available and the risk of spread of disease caused by those pathogens is high. Examples of RG4 human pathogens listed in Schedule 4 of HPTA
- Security clearance
- means a security clearance issued under section 34 of HPTA.
- Toxin
- A poisonous substance that is produced or derived from a microorganism and can lead to adverse health effects in humans or animals. Human toxins are listed in Schedule 1 and Part 1 of Schedule 5 in the HPTA.
Guidelines
1. The Canada Border Services Agency (CBSA) collaborates with the Public Health Agency of Canada (PHAC), Canadian Food Inspection Agency (CFIA) and Global Affairs Canada (GAC) in the administration of the Human Pathogens and Toxins Act (HPTA), the Human Pathogens and Toxins Regulations (HPTR), the Health of Animals Act (HAA), the Health of Animals Regulations (HAR), Plant protection Act (PPA), the Plant Protection Regulations (PPR), and Export and Import Permits Act (EIPA).
2. PHAC is responsible for the administration and enforcement of the HPTA, the HPTR and the administration of specific sections of the HAR.
3. CFIA is responsible for the administration and enforcement of the HAA, the HAR, the PPA and the PPR.
4. GAC is responsible for the administration and enforcement of the Export Control List (ECL) under the EIPA.
Importing pathogens or toxins
5. Importers carrying potentially infectious substances or other biological substances on their person or in their luggage must declare those substances to the CBSA and present document(s), which identifies these substances, such as a package label, manufacturers/suppliers forms or certificates, and:
- PHAC licence (see appendix A for an example of PHAC's licence) under the HPTA/HAR and/or
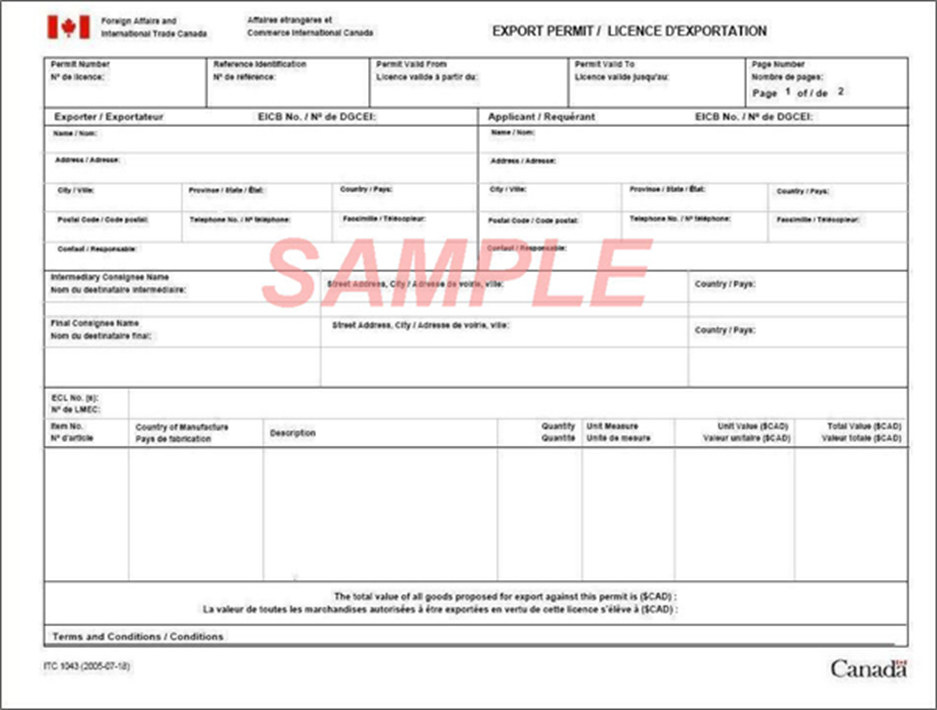
- CFIA import permit under the HAR/PPR (see appendix A for an example of CFIA permit).
6. Licences and permits are obtained via PHAC and/or the CFIA.
7. If you intend to import, you will need to contact:
- The PHAC's Biosafety and Biosecurity Licensing Program to obtain a:
- Pathogen and Toxin Licence issued under the section 18 of the HPTA for human pathogens and toxins, and/or
- Pathogen and Toxin Licence issued under the section 160 of the HAR for:
- cultures of indigenous terrestrial animal pathogens or part of one;
- purified or synthesized samples of toxins derived from indigenous terrestrial animal pathogens; and
- indigenous terrestrial animal pathogens or part of one carried in or on a substance other than an animal, animal product, animal by-product, or other organism (e.g., human specimens, plant tissues, food matrices such as oatmeal or mashed potatoes, environmental samples or quality controls).
- The CFIA to obtain import permit(s) for:
- any terrestrial or aquatic animal pathogen or part thereof in an animal product or by-product (such as tissue, blood, fetal bovine serum, etc.);
- any terrestrial or aquatic animal pathogens or part thereof in a live animal;
- all non-indigenous terrestrial animal pathogens or part thereof (i.e. Foreign Animal Disease (FAD) and Emerging Animal Disease (EAD) agents);
- all non-human primate (NHP) products and by-products (such as feces, serum, cell lines, etc.);
- all aquatic animal pathogens;
- all bee pathogens;
- specified Risk Materials (SRM) and other products that can carry prions; and
- plant pathogens.
8. If a person is carrying a human pathogen or toxin, the carrier must also meet the obligations under the Transportation of Dangerous Goods Regulations (packaging, labelling, documentation, markings).
9. Important note: Please refer to the ePATHogen - Risk Group Database to query the classification of microorganism or toxin. For other questions related to the risk group classification of biological agents, or which Agency it is regulated by, please contact PHAC at pathogens.pathogenes@phac-aspc.gc.ca and/or CFIA at permission@inspection.gc.ca.
- On the ePATHogen - Risk Group Database, the column "CFIA" identifies some of the agents that are identified as Foreign and Emerging Animal Disease (FAD/EAD) agents that are under CFIA's authority.
- Importation of a terrestrial animal pathogen in an animal, animal product or by-product, is also under CFIA's authority if identified with an Animal Classification of RG2 or higher, regardless if it is identified as "yes" under the CFIA column.
Exporting pathogens or toxins
10. Exporters wishing to export pathogens and toxins listed on the ECL must obtain a permit from GAC, and for pathogens and toxins regulated under HPTA, exporters must obtain a licence from PHAC.
- Global Affairs Canada regulates the export of human and animal pathogens listed on the ECL under the EIPA:
- GAC's export permits are required for the exportation of all goods and technology listed in the ECL: Group 7 of the ECL includes items that could be used to produce chemical and biological weapons or that are chemical weapons, precursors to chemical weapons, or listed pathogens or toxins.
- Section 7-13.1,7-13.2 and 7-13.3 of Group 7 of the ECL (please refer to A Guide To Canada's Export Controls for details) contain lists of pathogens and toxins that require export permit from GAC.
- For more information about requirements under the EIPA please refer to Memorandum D19-10-3 - Administration of the Export and Import Permits Act (Exportations) or visit GAC's website (Export Controls)
- HPTA establishes that it is prohibited for a person to knowingly export a human pathogen or toxin unless a licence has been issued. This prohibition does not overlap with the separate export controls on human pathogens and toxins included on the ECL, under the EIPA.
11. The exporter must follow the exporting requirements under Section 95 of the Customs Act. The exporter must present a copy of the export permit or licence to the CBSA within the time frames specified on the permit and at the place specified in the permit authorizing the exportation. If no place is specified in that permit, it must be presented at the export reporting office located closest to the place of exit of goods from Canada.
12. As described in section 4.(1)(d) of the HPTR, "(d), a person who intends to export a human pathogen or toxin must, before they export it, take reasonable care to be satisfied that the intended recipient will conduct any activities in respect of the human pathogen or toxin in accordance with any applicable biosafety and biosecurity standards and policies in the foreign jurisdiction." Further details regarding this statement of the HPTR can be found in section 23.5.3 Exportation of Pathogens from Canada in the Canadian Biosafety Handbook.
Exemptions
13. There are two specific cases under the HPTA (Section 7 (2) of the HPTA), where exemptions from the licensing requirement apply; the requirement for a PHAC licence does not apply to:
- Activities covered by other regulatory regimes:
- the export of human pathogens or toxins authorized under the Export and Import Permits Act, 1985
- Certain persons in the course of their work:
- an inspector or analyst carrying out their functions under the HPTA;
- a peace officer carrying out their functions under any federal or provincial law or a person providing assistance to a peace officer;
- any person who collects samples in the course of their employment, outside a facility in which controlled activities are authorized, for laboratory analysis or diagnostic testing;
- any person working under a federal or provincial Act, in exigent circumstances.
14. PHAC's Statement of Administrative Intent (SAI) clarifies these and other exemptions from the licensing requirements of the HPTA and HPTR.
Exclusions
15. An HPTA Pathogen and Toxin Licence is not required for:
- a human pathogen or toxin that is in an environment in which it naturally occurs, if it has not been cultivated or intentionally collected or extracted, including but not limited to a human pathogen or toxin that:
- is in or on a human suffering from a disease caused by that human pathogen or toxin;
- has been expelled by a human suffering from a disease caused by that human pathogen or toxin; and
- is in or on a cadaver, a body part or other human remains.
- a drug in dosage form whose sale is permitted or otherwise authorized under the Food and Drugs Act or a human pathogen or toxin contained in such a drug.
Role of the CBSA
16. The border services officers (BSO) will verify the:
- accuracy of import or export permits/licences (permit/licence number, validity dates, exporter name, quantities, etc.) issued by PHAC, CFIA or GAC;
- validity of Pathogen and Toxin Licences issued by PHAC to ensure that they correspond with the licence format as identified below:
Licence Type Format Maximum Validity period RG2 Licence L-R2-#####-YY-AA 5 years RG3 Licence L-R3-#####-YY-AA 3 years RG4 Licence L-R4-#####-YY-AA 1 year SSBA Toxin Licence L-ST-#####-YY-AA 3 years
Please note that regulated parties have the option to download a condensed version of their Pathogen and Toxin Licence issued by PHAC, which displays no sensitive information directly from the Biosecurity Portal.
17. Under Section 101 of the Customs Act, the CBSA may detain shipments suspected of non-compliance (e.g., permit(s)/license is missing, or information on the permit or licence is missing or inconsistent, supplemental document (e.g. zoosanitary certificate) required as a condition of permit) and notify PHAC, CFIA or GAC for admissibility recommendations and possible enforcement actions if applicable.
Penalties
18. Importing or exporting a pathogen or toxin subject to legislation without licence or permit(s) is an offence subject to fines and potential imprisonment. For more information, please refer to the:
- Offences and Punishment part of the HPTA (sections 53 to 58);
- Offences and Punishment part of the of the HAA (sections 65 to 73);
- Offence and Punishment part of the EIPA (section 19).
19. The Administrative Monetary Penalty System (AMPS) authorizes the CBSA to impose monetary penalties for non-compliance with the Customs Act, the Customs Tariff Act and the regulations enacted under these Acts, as well as contraventions of the terms and conditions of licensing agreements and undertakings.
20. Importers can find more information concerning AMPS in Memorandum D22-1-1, Administrative Monetary Penalty System.
21. BSOs may also seize items for contravening the Customs Act.
Appendix A: Samples of permits and licences
PHAC: licence sample
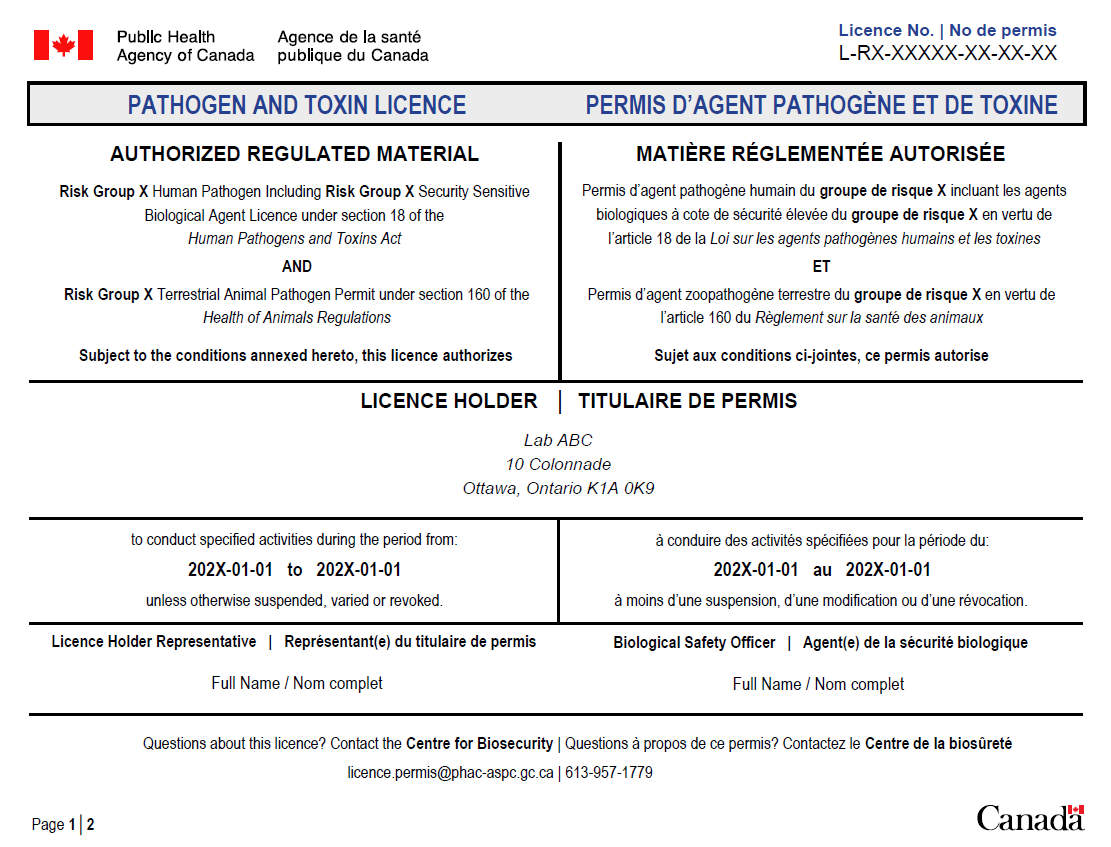
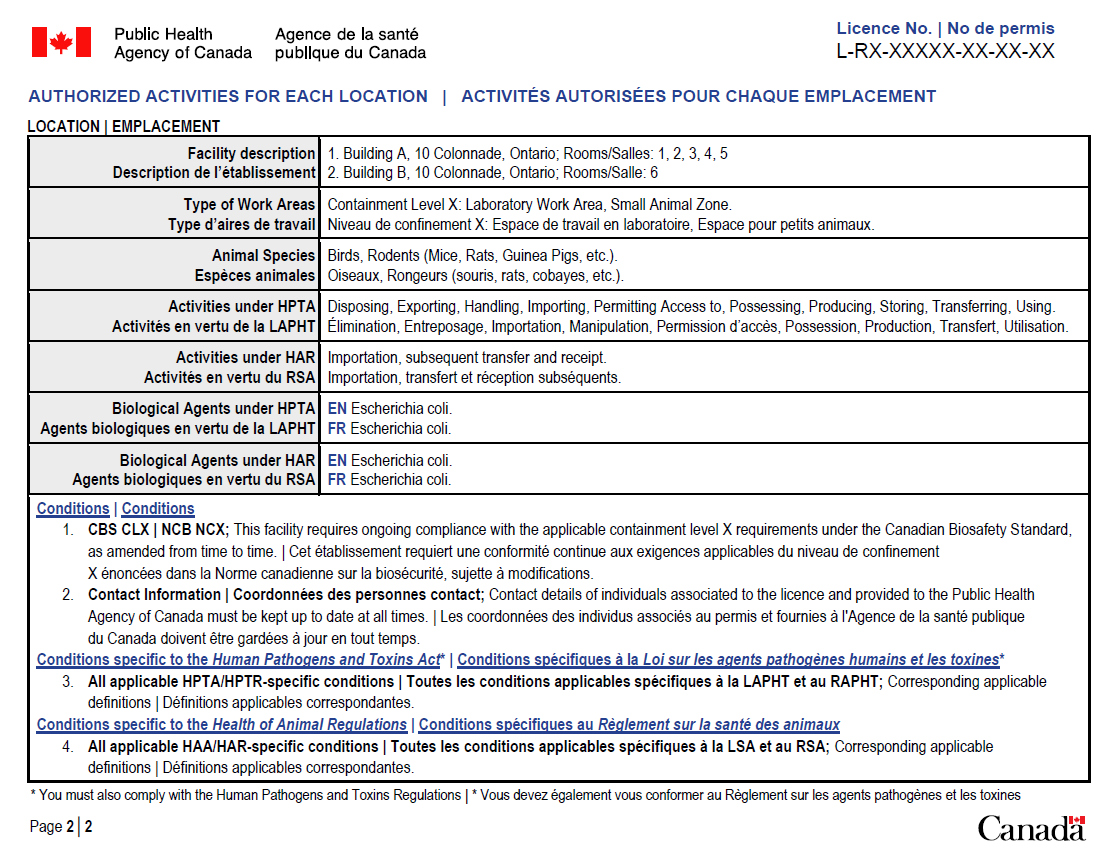
CFIA: import permit sample



GAC: export permit sample
References
Applicable legislation
- Human Pathogens and Toxins Act
- Human Pathogen and Toxins Regulations
- Health of Animals Act
- Health of Animal Regulations
- Export and Import Permits Act
- Export Control List
- Plant Protection Act
- Plant Protection Regulations
- Food and Drugs Act
Superseded memoranda D
N/A
Issuing office
Program and Policy Management Division
Commercial Program Directorate
Commercial and Trade Branch
Contact us
Canadian Biosafety Standards and Guidelines introduce additional resources, including Biosafety directives and advisories. Please consult Biosafety directives and advisories before importing and/or exporting pathogens and toxins:
- Biosafety directives provide regulated parties with the customized containment requirements for activities with a specific pathogen or group of pathogens when the containment level does not align with the risk group.
- Biosafety advisories are developed when the data obtained from a risk assessment of a new or emerging pathogen of interest indicates that new physical or operational requirements are required to work with the pathogen safely.
For more information regarding PHAC's Pathogen and Toxin Licensing Program, contact the Licensing Program by:
- Phone at 613-957-1779
- E-mail at licence.permis@phac-aspc.gc.ca
For more information on pathogen import permits issued by the CFIA, contact:
- National Import Service Centre (open 7:00 am to 3:00 am EST) by:
- Phone: 1-800-835-4486
- Facsimile: 1-613-773-9999
- E-mail: cfia.nisc-csni.acia@inspection.gc.ca
- Centre of Administration for Permissions (open from 7:00 am to 7:00 pm EST) by:
- Phone: 1-800-442-2342 or 613-773-2342
- E-mail: Permission@inspection.gc.ca
For more information regarding export permits issued by GAC under the EIPA, contact:
Export Controls Division (TIE)
Global Affairs Canada
125 Sussex Drive
Ottawa ON K1A 0G2
Telephone: (343) 203-4331
Facsimile: (613) 996-9933
E-mail: tie.reception@international.gc.ca
For more information regarding the CBSA's programs and services, please contact the Border Information Service (BIS) line. Within Canada, you can call BIS toll-free at 1-800-461-9999. From outside Canada, please call 204-983-3500 or 506-636-5064 (long-distance charges will apply). Agents are available Monday to Friday (08:00 – 16:00 local time, except holidays). TTY is also available within Canada at 1-866-335-3237.
Related links
Memorandum D19-10-3 - Administration of the Export and Import Permits Act (Exportations)
- Date modified: